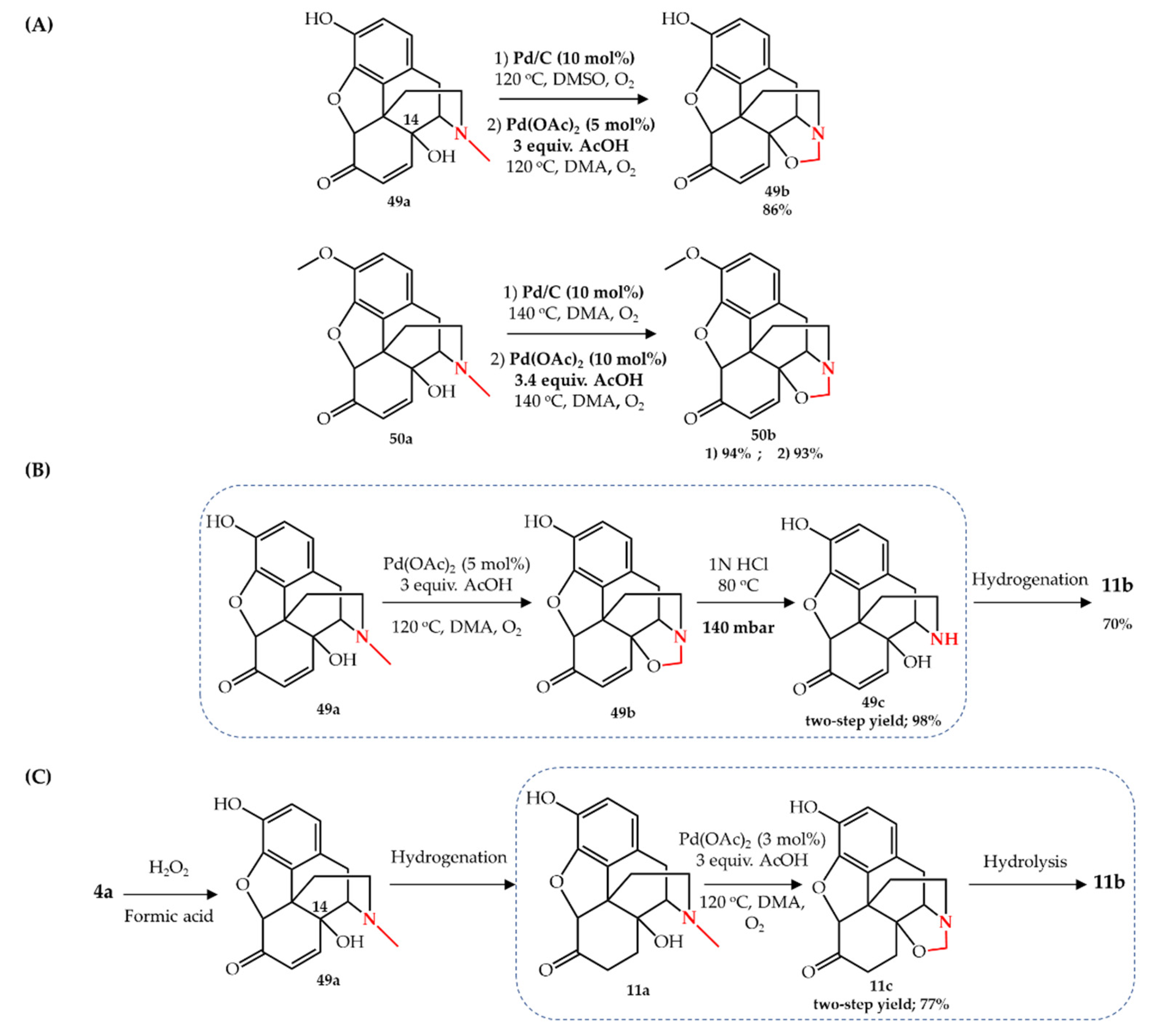
![For the reaction: 2A + B → A2B , the rate = k[A][B]^2 with k = 2.0 × 10^-6 mol^-2 L^2 s^-1 . Calculate the initial rate of the reaction when [A] = For the reaction: 2A + B → A2B , the rate = k[A][B]^2 with k = 2.0 × 10^-6 mol^-2 L^2 s^-1 . Calculate the initial rate of the reaction when [A] =](https://d1hhj0t1vdqi7c.cloudfront.net/v1/TVhJa2FqVWNSMFk=/sd/)
For the reaction: 2A + B → A2B , the rate = k[A][B]^2 with k = 2.0 × 10^-6 mol^-2 L^2 s^-1 . Calculate the initial rate of the reaction when [A] =

If `NaCl` is doped with `10^(-3)` mol% of `SrCl_(2)`, what is the concentration of cation vacanc... - YouTube
123 The molar solubility of CaF2 (Ks5.3 x10 11) in 0.1 M solution of NaF will be Co5.3 x 10 11 mol L 1(2) 5.3 × 10 8 mol L 1A) E) S.3 × 10 9 mol L 1くが(141) 5.3 × 10 10 mol L 1"卡
![Solubility product of AgCl is 1.8 × 10^-10 . Calculate its molar solubility and solubility in g/L. [Molar mass of AgCl is 143.5 g mol^-1 ] Solubility product of AgCl is 1.8 × 10^-10 . Calculate its molar solubility and solubility in g/L. [Molar mass of AgCl is 143.5 g mol^-1 ]](https://dwes9vv9u0550.cloudfront.net/images/5064616/add4819a-6281-4659-ab5c-8471f2633afa.jpg)
Solubility product of AgCl is 1.8 × 10^-10 . Calculate its molar solubility and solubility in g/L. [Molar mass of AgCl is 143.5 g mol^-1 ]

Oxidation of secondary alcohols using 10 mol% lanthanum triflate as... | Download Scientific Diagram

How many atoms or molecules are present in 1.0 mol of Au? How am I able to find the answer? | Socratic

Quantos atomos de oxigenio existem em 0,10 mol de glicose (C6H1206)? Constante de avogadro: 6,x - Brainly.com.br

Site-selective photocatalytic functionalization of peptides and proteins at selenocysteine | Nature Communications