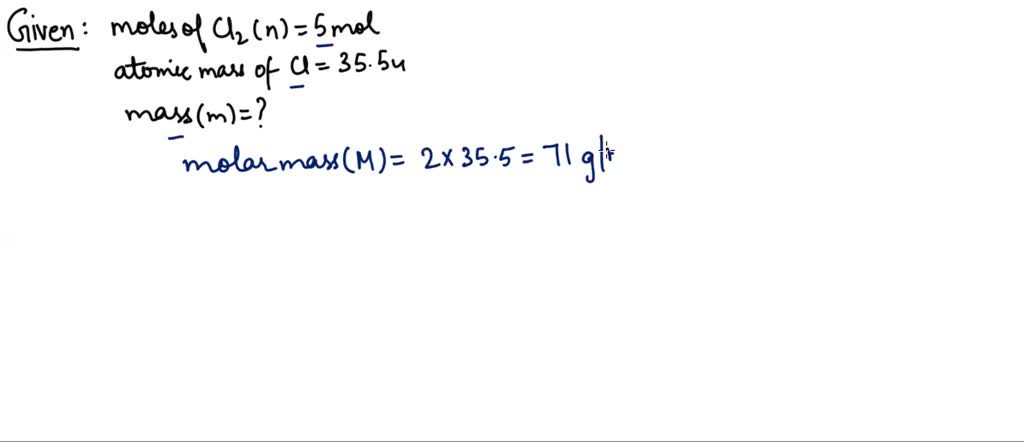5 moles of SO2 and 5 moles of O2 are allowed to react to form SO3 in a closed vessel. At the equilibrium stage, 60% SO2 is used up. The total number

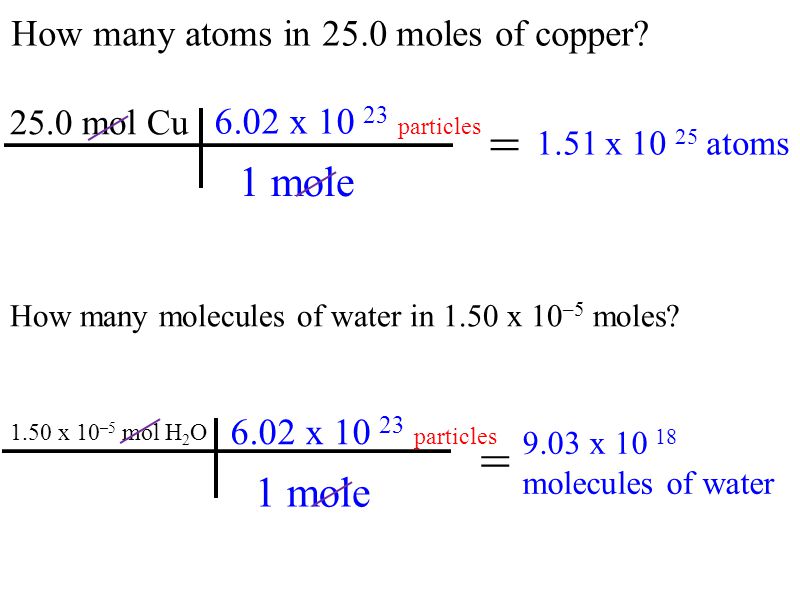
Question Video: Calculating the Moles of a Reactant Consumed in a Reaction Given the Moles of a Second Reactant | Nagwa
The temperature of 5 moles of a gas at constant volume is changed from 100 celcius to 120 degree celcius.The change in internal energy is 80 J.The total heat capacity of the
SOLVED: 10 What is the mass of 5 moles of hydrogen (H)? (10 Points) 1.008 grams 2.016 grams 4.032 grams 5.040 grams

5 mole of an ideal gas expand isothermally and irreversibly from a pressure of 10 atm to 1 atm against a constant external pressure of 1 atm. w irr at 300K is :