PDF) Bassanite (CaSO4·0.5H2O) dissolution and gypsum (CaSO4·2H2O) precipitation in the presence of cellulose ethers
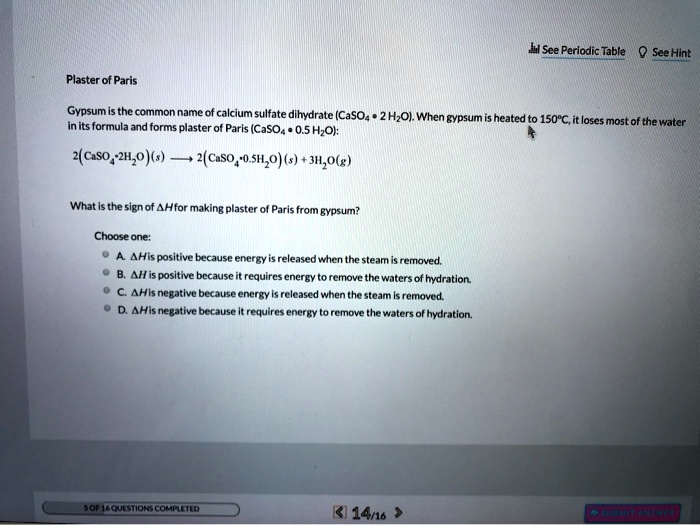
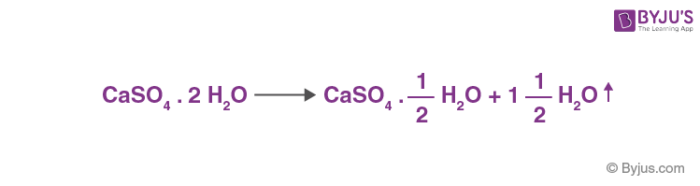
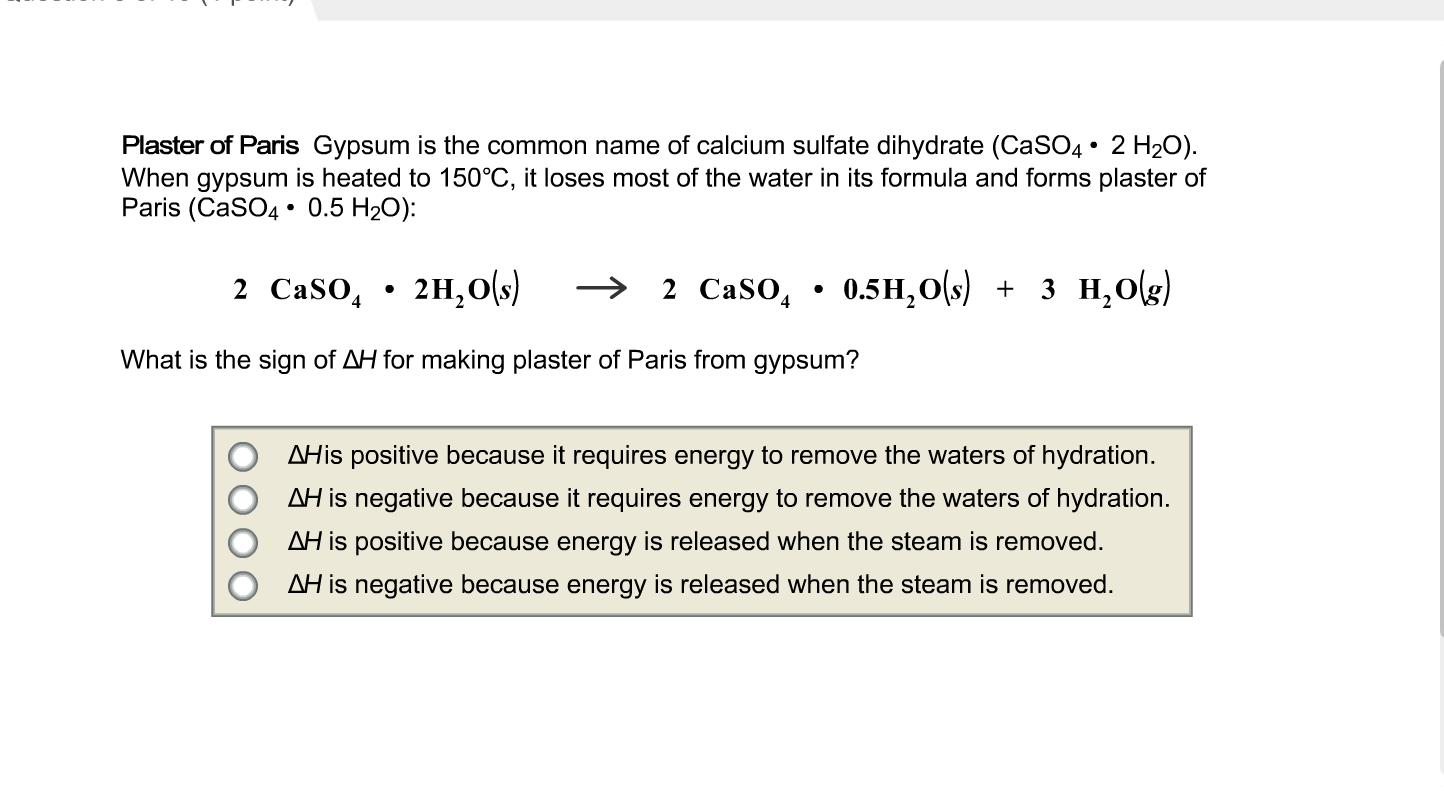
SOLVED: H See Perlodic Table Sce Hint Plaster of Paris Gypsum the common name calcium sulfate dihydrate (CaSOa- 2 HzOl. When gypsum heated 150*C,it loses [ most of the water inits formula

The effect of additives on the hydration of CaSO4·0.5H2O: A synchrotron X-ray diffraction study - ScienceDirect

Thermodynamic Modeling of Calcium Sulfate Hydrates in the CaSO4–H2O System from 273.15 to 473.15 K with Extension to 548.15 K | Journal of Chemical & Engineering Data
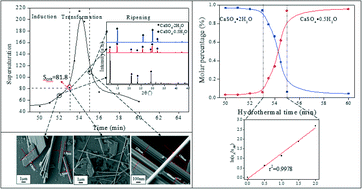
Supersaturation-induced hydrothermal formation of α-CaSO4·0.5H2O whiskers - CrystEngComm (RSC Publishing)
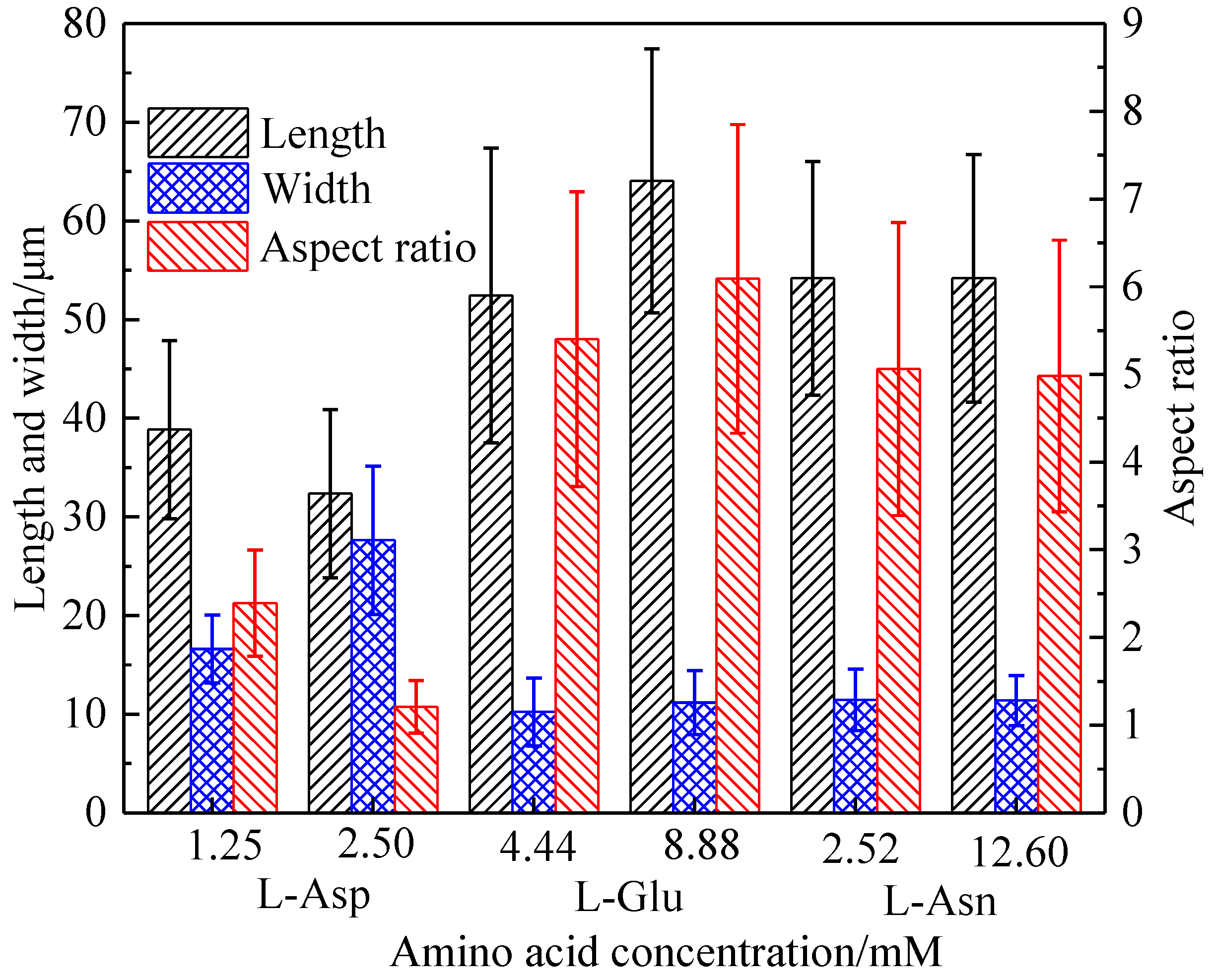
Crystals | Free Full-Text | Effect of Molecular Structure of Organic Acids on the Crystal Habit of α-CaSO4·0.5H2O from Phosphogypsum

SOLVED: Gypsum is the common name of calcium sulfate dihydrate which has the formula CaSO4· 2 H2O When gypsum is heated to 150^∘C, it loses most of the water in its formula

Crystals | Free Full-Text | Influence of Alkyl Trimethyl Ammonium Bromides on Hydrothermal Formation of α-CaSO4·0.5H2O Whiskers with High Aspect Ratios

Formation and Transformation of Five Different Phases in the CaSO4−H2O System: Crystal Structure of the Subhydrate β-CaSO4·0.5H2O and Soluble Anhydrite CaSO4 | Chemistry of Materials

PDF) Thermal behaviour and kinetics of dehydration in air of bassanite, calcium sulphate hemihydrate (CaSO4· 0.5 H2O), from X-ray powder diffraction | Paolo Ballirano - Academia.edu
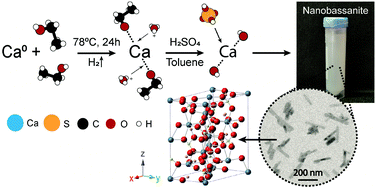
Synthesis of high surface area CaSO4·0.5H2O nanorods using calcium ethoxide as precursor - Chemical Communications (RSC Publishing)