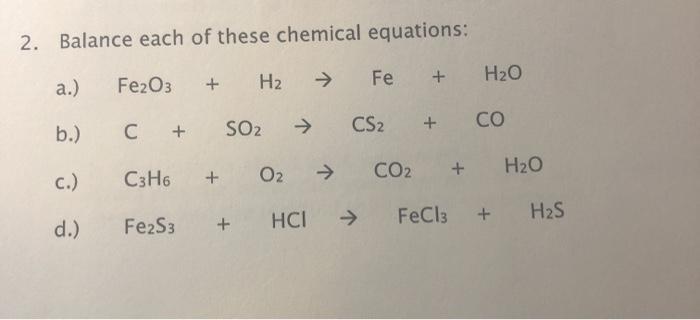
See answer: Which is the correct equilibrium constant expression for the following reaction? Fe2O3 (s) 3H2 - Brainly.com
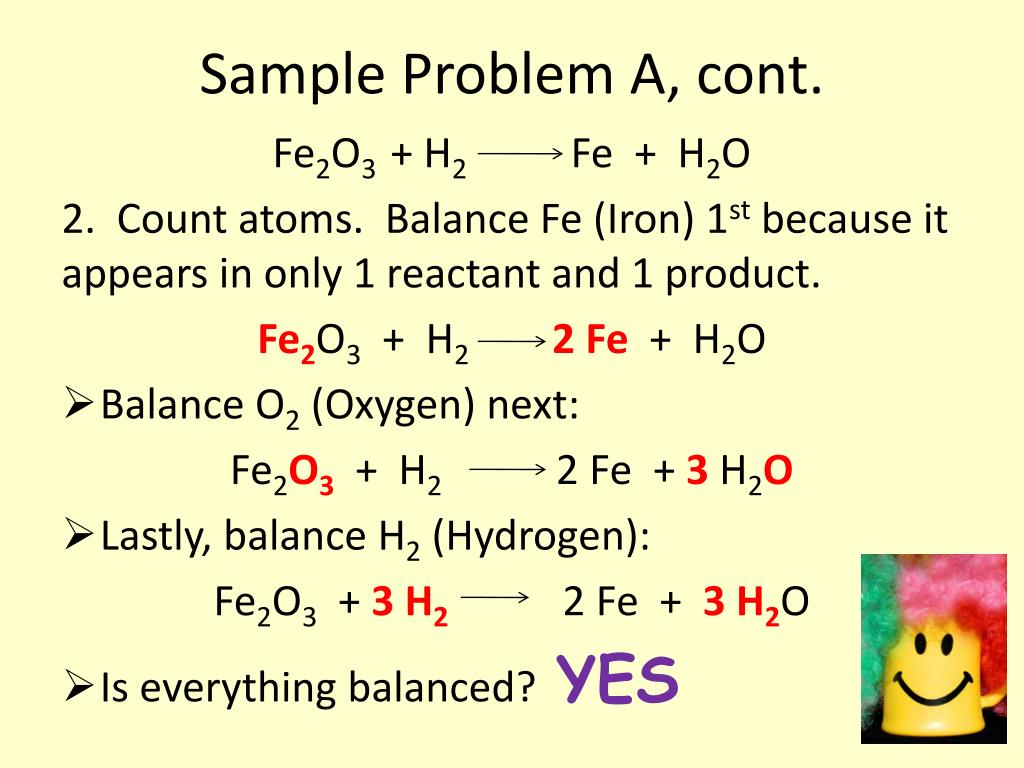
PPT - Friday, Aug. 30 th : “A” Day Tuesday, Sept. 3 rd : “B” Day Agenda PowerPoint Presentation - ID:3916169

В схеме реакции Fe2O3+H2➡Fe+H2O Fe2O3+H2➡Fe+H2O подберите коэффициенты методом электронного баланса . Укажите окислитель и восстановитель - вопрос №2206623 - Учеба и наука

Balance the equation- rn Fe + O2 + H2O ---- Fe2O3 - Science - Physical and Chemical Changes - 11497 | Meritnation.com
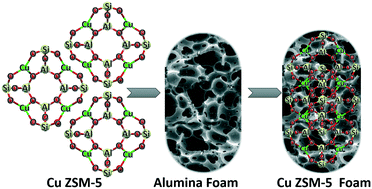
Selective catalytic reduction of NO over hierarchical Cu ZSM-5 coated on an alumina foam support - Reaction Chemistry & Engineering (RSC Publishing)
![SOLVED: Determine the equilibrium constant for the reaction between iron (III) oxide and hydrogen. Fe2O3 (s) + 3H2 (g) 2Fe (s) + 3H2O (g) Group of answer choices Kc = [H2O]3 / [ SOLVED: Determine the equilibrium constant for the reaction between iron (III) oxide and hydrogen. Fe2O3 (s) + 3H2 (g) 2Fe (s) + 3H2O (g) Group of answer choices Kc = [H2O]3 / [](https://cdn.numerade.com/ask_previews/d4d6e0e8-f43e-406e-85e1-9586a85b0f23_large.jpg)