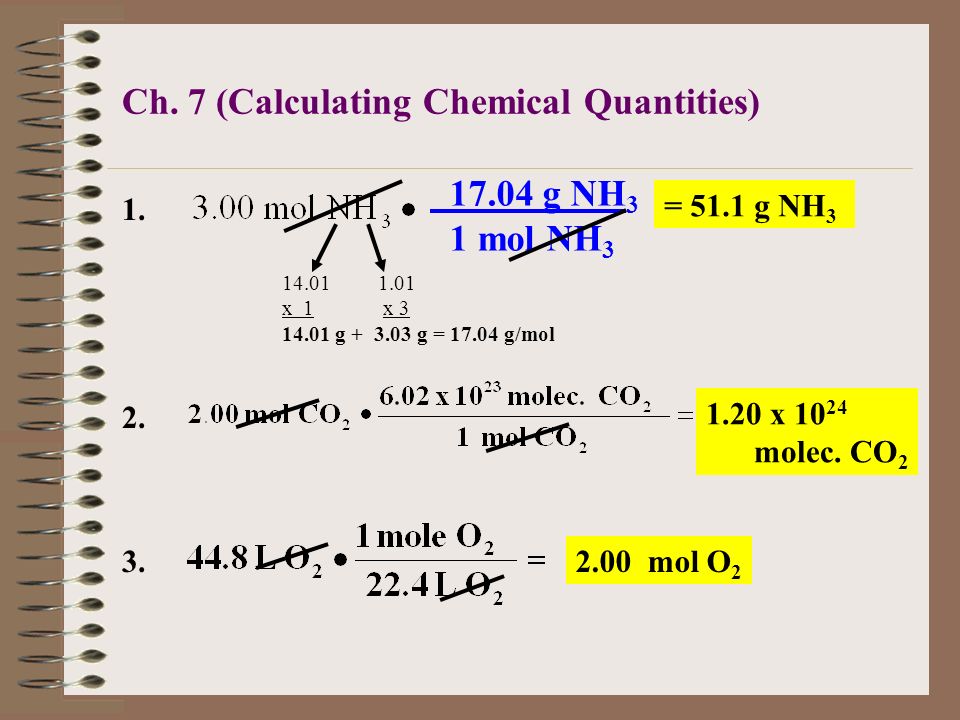
Bisphenol A, molecular formula: C 15 H 16 O 2, molar mass is 228.29 g/mol. | Download Scientific Diagram
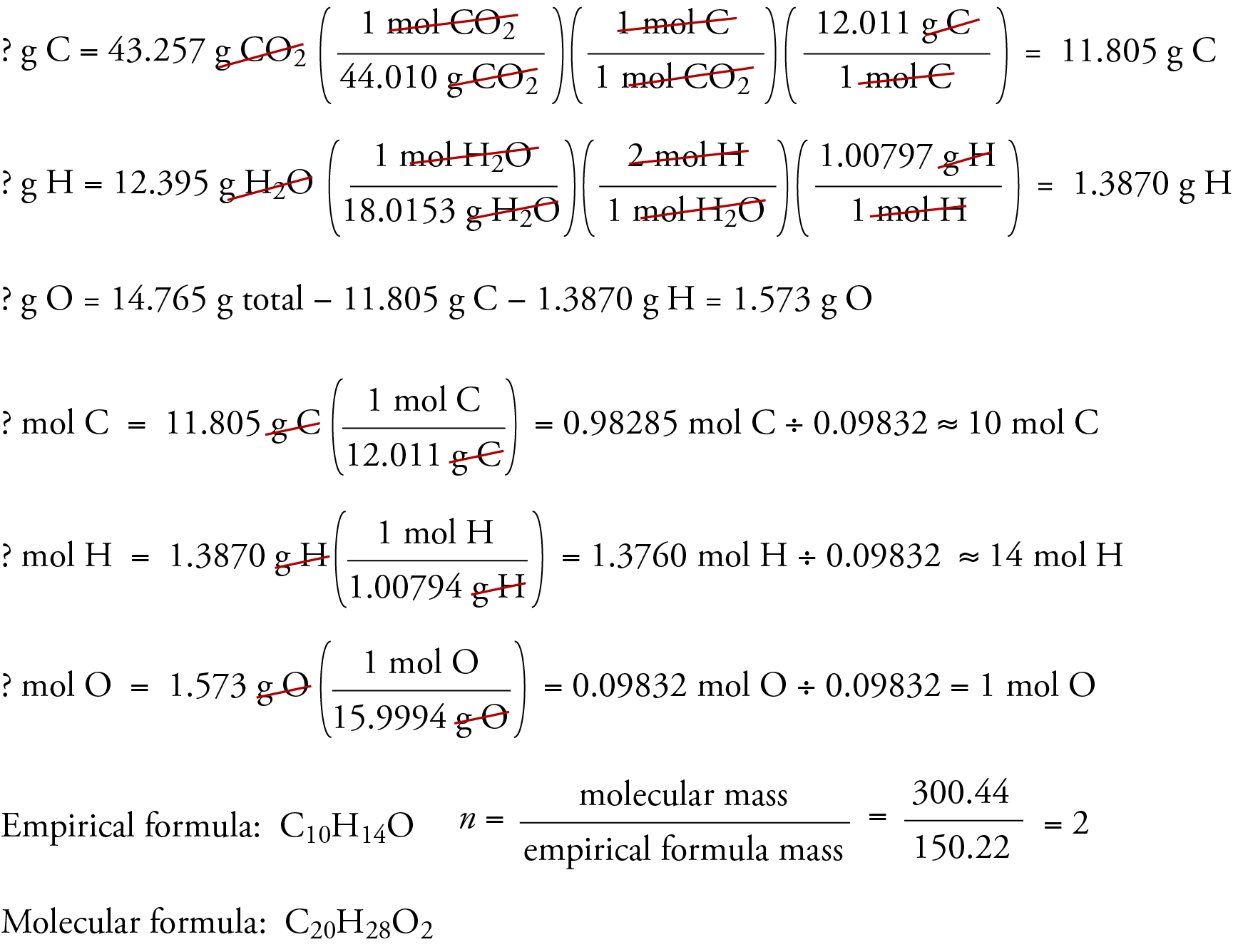
Empirical and molecular formulas for compounds that contain only carbon and hydrogen (C a H b ) or carbon, hydrogen, and oxygen (C a H b O c ) can be determined with a process called combustion analysis. The steps for this procedure are

Question Video: Calculating the Mass of Solute Needed to Prepare a Solution with a Desired Concentration and Volume | Nagwa

SOLVED: Determine the number of moles of oxygen atoms in each sample. a. 4.88 mol H2O2 b. 2.15 mol N2O c. 0.0237 mol H2CO3 d. 24.1 mol CO2
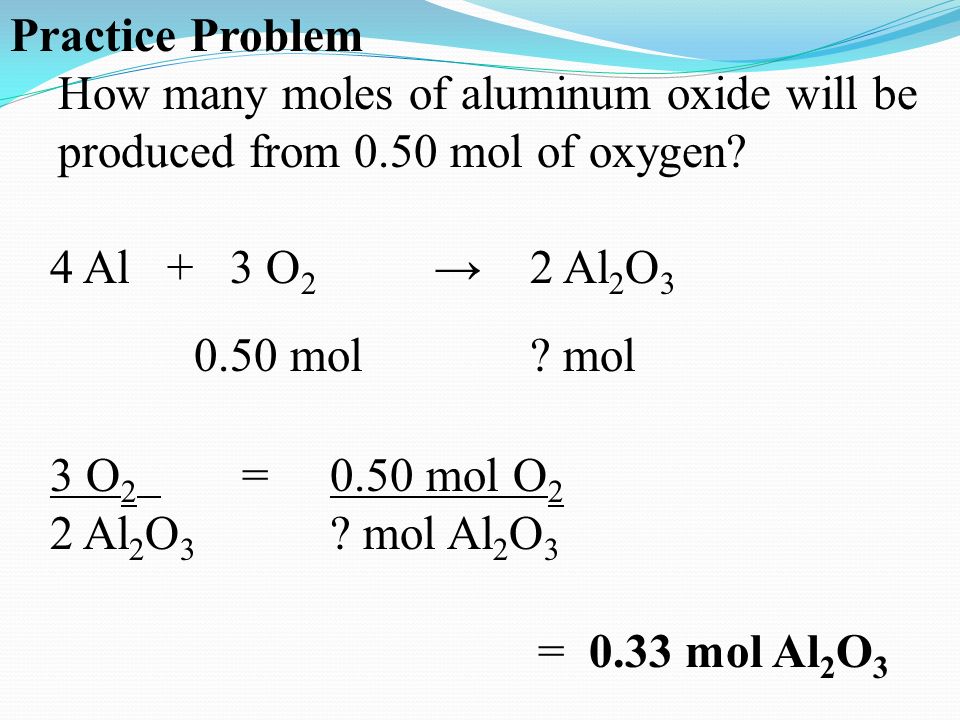
Practice Problem How many moles of aluminum oxide will be produced from 0.50 mol of oxygen? 4 Al + 3 O 2 → 2 Al 2 O mol? mol 3 O 2 = 2 Al 2 O ppt download
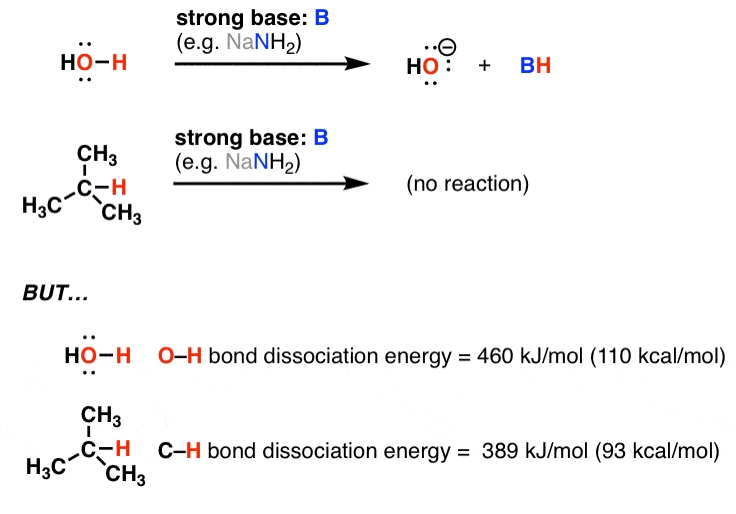
Objective: Define empirical formula, and explain how the term applies to ionic and molecular compounds
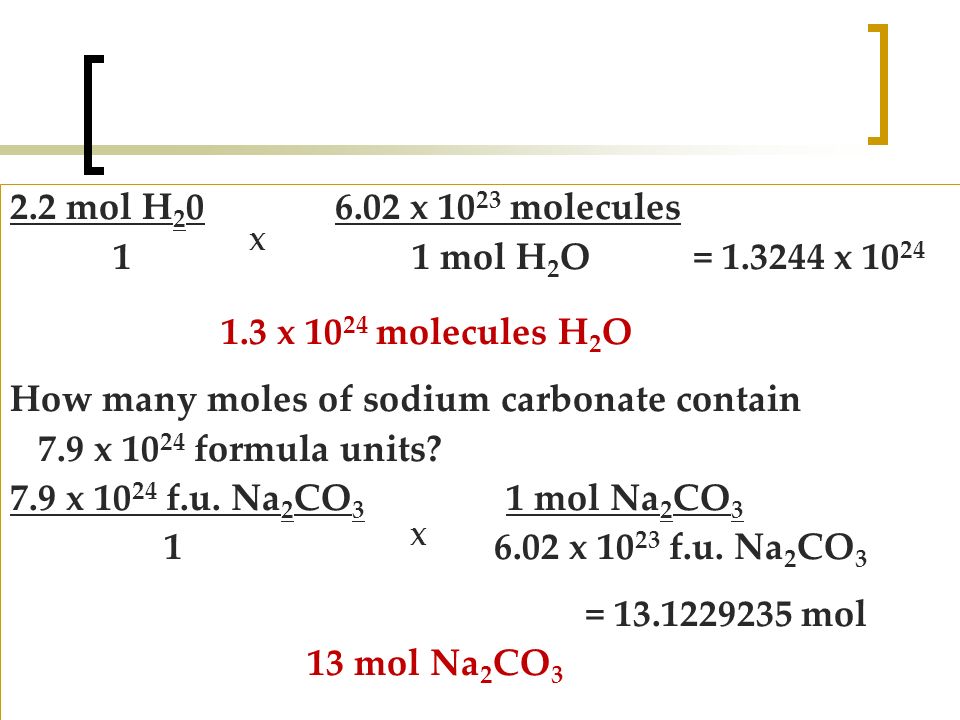
The Mole & Chemical Quantities. The Mole Mole-the number of particles equal to the number of atoms in exactly 12.0 grams of carbon mol = 6.02 x. - ppt download